Description
Synonym: Hydroxylammonium chloride
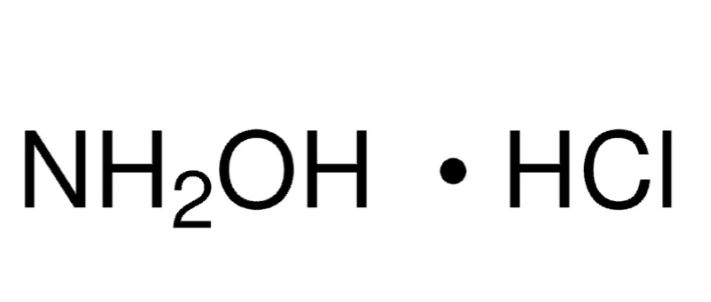
Linear Formula: NH2OH · HCl
General description
Hydroxylamine hydrochloride (Hydroxylammonium chloride) participates in the synthesis of 1,2,4-oxadiazoles, secondary amides and tertiary amides.
Hydroxylamine hydrochloride (NH2OH.HCl) is a hygroscopic salt widely used as a reactant in electrophilic substitution reactions, oxidation, and reduction reactions. It is also used in the synthesis of nitriles, pyrazoles, isoxazoles, nitrones, and pyridine.
Application
Hydroxylamine hydrochloride can be used as a reactant:
- in the synthesis of primary amides from aldehydes in the presence of cesium carbonate (Cs2CO3) as a catalyst.
- in the conversion of alicyclic /aliphatic carbonyl compounds and the aromatic aldehydes into corresponding oximes.
- in the one-pot synthesis of nitriles from aldehydes in the presence of sodium sulfate (anhyd) and sodium bicarbonate catalysts.
- It can also be used as a reducing agent in the preparation of single-layer reduced graphene oxide (RGO) sheets and films.
Biochem/physiol Actions
MAO inhibitor; inhibits platelet aggregation.
PROPERTIES
vapor pressure
0.001 hPa ( 50 °C)
Pack Size
100g; 500g; 1kg
product line
ReagentPlus®
Assay
99%
form
crystalline
technique(s)
inhibition assay: suitable
pH
2.5-3.5 (20 °C, 50 g/L)
mp
155-157 °C (dec.) (lit.)
density
1.67 g/mL at 25 °C (lit.)
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany


![Hydrochloric acid 2 mol/l (2 N) Titripur® [1 Litre]](https://idealmedical.co.za/wp/wp-content/uploads/2020/07/256-Hydrochloric-acid-200x200.jpg)

Reviews
There are no reviews yet.