Description
Linear Formula: KClO3
Molecular Weight: 122.55
General description
Crystals of potassium chlorate belong to the monoclinic crystal system. On heating above 400°C, it degrades to give potassium chloride and potassium perchlorate. It has strong oxidizing properties. On exposure to X-rays, oxygen-17 enriched KClO3 generates O3– radical, which was investigated by ESR (Electron Spin Resonance). Irradiation of potassium chlorate with X-ray causes the degradation to generate chloride, chlorite, hypochlorite and occluded oxygen. Significant quantities of dichlorine hexoxide and a trace of chlorine dioxide were also produced.
Application
Potassium chlorate may be used in the preparation of graphite oxide (GO).
PROPERTIES
grade
puriss.
Pack Size
1kg
Assay
99-101%
form
powder or crystals
impurities
≤0.001% heavy metals (as Pb)
pH
5-6.5 (25 °C, 61.3 g/L)
mp
356 °C (lit.)
anion traces
chloride (Cl–): ≤100 mg/kg
sulfate (SO42-): ≤100 mg/kg
cation traces
As: ≤2 mg/kg
Ca: ≤50 mg/kg
Fe: ≤30 mg/kg
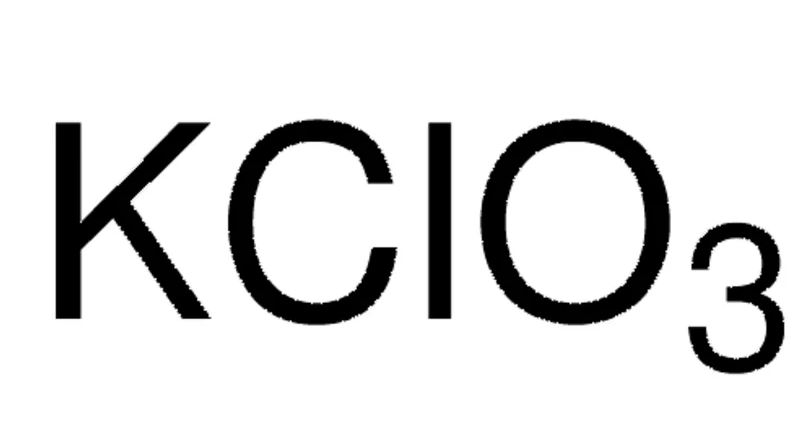




Reviews
There are no reviews yet.