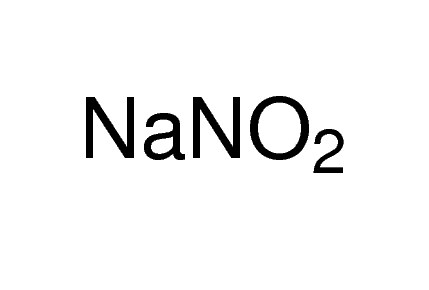
Description
General description
Application
Co-catalyst for catalytic oxidation of alcohols under aerobic, solvent-free conditions.
Sodium nitrite can be used as a reagent in:
- The Sandmeyer reaction for converting amines into diazo derivatives, and nitration reaction.
- The oxidative C-C bond formation reaction in the presence of oxygen as the terminal oxidant.
- The oxidative carbonitration of alkenes in the presence of K2S2O8.
It can also be used as a nitrating agent in organic synthesis. For example, NaNO2 is used in direct C-H nitration, ipso-nitration, and nitration via transition metal-catalyzed cross-coupling reaction.
PROPERTIES
Pack Size
500g
Agency
reag. Ph. Eur.
Assay
≥99.0% (manganometric)
form
solid
potency
85 mg/kg LD50, oral (Rat)
impurities
≤0.01% Insoluble matter
pH
9 (20 °C, 100 g/L in H2O)
mp
280 °C (decomposition)
solubility
820 g/L
density
2.1 g/cm3 at 20 °C
bulk density
1200 kg/m3
anion traces
chloride (Cl–): ≤0.005%
sulfate (SO42-): ≤0.005%
cation traces
Ca: ≤0.002%
Fe: ≤0.001%
K: ≤0.001%
heavy metals (as Pb): ≤0.001%
storage temp.
2-30°C

![Universal indicator solution uniLAB, Merck [100ml]](https://idealmedical.co.za/wp/wp-content/uploads/2020/08/276-pH-indicator-soln-300x300.jpg)

Reviews
There are no reviews yet.