Description
Mode of Action
Thioglycollate and L-Cystine in the medium reduce the redox potential of the culture medium in order to create an anaerobic atmosphere. In addition, mercury and other heavy metal compounds are inactivated by these agents. The content of agar further reduces a rapid diffusion of oxygen through the medium, but may lead to a slight turbidity in larger volumes (filled bottles). Resazurin indicates the reduction potential of the medium. An increased concentration of oxygen is indicated by a color change to pink.
Typical Composition
Casein Peptone 15 g/L
Yeast extract 5 g/L
Glucose Monohydrate 5.5 g/L
NaCl 2.5 g/L
L-Cystine 0.5 g/L
Sodium Thioglycollate 0.5 g/L
Resazurin 1 mg/L
Agar 0.75 g/L
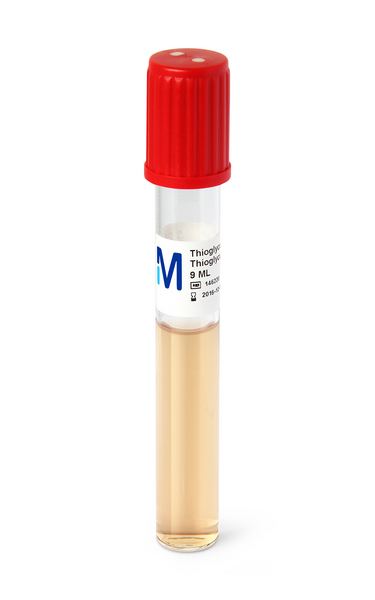
The appearance of the medium is clear to slightly turbid and yellowish. The pH value is in the range of 6.9-7.3.
The medium can be adjusted and/or supplemented according to the performance criteria required.
Application and Interpretation
The broth medium should be equilibrated to room temperature before use.
The surface of the containers, including the septum below the protective cap, is not sterile. Therefore, please be aware about a risk of secondary contamination due to handling. In order to reduce the risk of secondary contamination by defect glass containers or handling the following recommendations may be helpful:
• Please control each single container for visible defects or turbidity. Do not use such containers.
• Please avoid the contamination of culture media by contact with skin or body fluids. Such contaminated media cannot be used anymore.
• In the case of negative pressure due to prior heatsterilization, the containers should be ventilated by sterile filter units before usage to avoid aspiration of potential contaminated air.
• The risk of transfer of microorganisms from the surface of the containers into the sterile culture medium can be minimized by disinfection of these surfaces followed by handling in sterile environments, e.g. isolators. The inoculation of the containers by sterile cannulas is safer than procedures which require opening of media bottles or tubes.
Media which contain ingredients of animal or human origin such meat extract must be considered potentially
infectious. After contact of such media a disinfection of the affected skin area is recommended.
Strictly anaerobic microorganisms such as Clostridium sporogenes are growing in the lower, yellowish part of the broth medium. The growth of facultative anaerobic microorganisms such as Staphylococcus aureus is distributed in the complete broth medium.
Aerobic microorganisms such as Pseudomonas aeruginosa are able to grow in the upper, oxidized part of Fluid
Thioglycollate Medium indicated by a slight pink color. Usually the incubation is performed under aerobic conditions. Not more than the upper half of the medium should have undergone a color change to pink indicative of oxygen uptake at the end of the incubation period.
Fluid Thioglycollate Medium is recommended forsterility testing of pharmaceutical products according to the European and US Pharmacopoeia. According to the Pharmacopoeia a membrane filtration method should be performed wherever possible, but also direct inoculation methods are possible.
Fluid Thioglycollate Medium (article numbers 146385,146387, 146139 and 146220) – due to their closure
system and filling volumes – are solely designed and tested for direct inoculation method. If a completely clear Fluid Thioglycollate Medium is required for direct inoculation, we offer Fluid Thioglycollate Medium Clear (article number 146341 and 146590). Fluid Thioglycollate Medium Clear contains a reduced Agar content, whereby the other part of agar is replaced by a clear gelling agent. This does not influence the growth promoting properties of the medium.
Note: For membrane filtration application, we recommend the use of our 100 mL Fluid Thioglycollate Medium (article number 146386 or 146406) or our 100 mL clear Fluid Thioglycollate Medium (article number 146456 and 146333).
For direct inoculation the amount of the inoculated sample material should not exceed 10% of the volume of Fluid Thioglycollate Medium. Fluid Thioglycollate Medium is incubated for 14 days at 30 to 35 °C and visually inspected for growth.
The sterility test is passed, if no growth is visible at the end of incubation. It is recommended to identify grown microorganisms in order to find out the origin of contamination and to implement corrective actions.
Storage and Shelf life
The product can be used for tests until the expiry date if protected from light and properly sealed at +2 °C to +25 °C.
The testing procedures as described on the CoA can be started up to the expiry date printed on the label.
Disposal
Please mind the respective regulations for the disposal of used culture medium (e.g. autoclave for 20 min at 121 °C, disinfect, incinerate etc.).
![Trypcase Soy Broth, TSB (Soybean-Casein Digest Medium); 100ml [Pack of 10]-crimp cap with septum](https://idealmedical.co.za/wp/wp-content/uploads/2020/06/096-sterility-media_1-300x300.jpg)
![Fluid Thioglycollate Medium acc EP+USP [Pack of 10]](https://idealmedical.co.za/wp/wp-content/uploads/2020/06/102-Fluid-Thiogy-300x300.jpg)
Reviews
There are no reviews yet.