Description
=99.9% (titration), abs., 100 mg/mL, 300 nm ≤0.015
Synonyms: Trizma® base, amino-2-(hydroxymethyl)-1,3-propanediol, 2-, 2-Amino-2-(hydroxymethyl)-1,3-propanediol, THAM, Tris base, Tris(hydroxymethyl)aminomethane, Trometamol
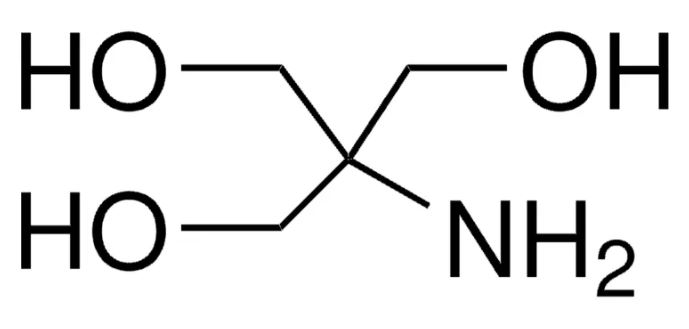
Linear Formula: NH2C(CH2OH)3
DESCRIPTION
Tris is an established basimetric standard and buffer used in biochemistry and molecular biology. It may be used by itself as a buffer or as a component of mixed buffer formulations, such as Tris-EDTA (TE) buffer, Tris-acetate-EDTA (TAE) buffer, Tris-borate-EDTA (TBE) buffer, etc. It is pure, essentially stable, relatively non-hygroscopic and has a high equivalent weight.
Application
Tris base has been used:
- As a component of lysis buffer for cell disruption
- In the preparation of TBE solution for PAGE (polyacrylamide gel electrophoresis)
- In studies of double stranded complexes of peptide nucleic acids (PNA) and their complementary DNA sequences, by use of anion exchange HPLC
- In capillary electrochromatography and UV analysis of tocopherols and tocotrienols
Quality
Contaminants: <0.001% PK and aldolase, each, <0.01% GOT, GPT, MDH and myokinase, each
PROPERTIES
description
aminopeptidase substrate
Assay
99.9% (titration)
packaging
pkg of 1 kg
manufacturer/tradename
Roche
useful pH range
7-9
pKa (25 °C)
8.1
bp
219-220 °C/10 mmHg (lit.)
mp
167-172 °C (lit.)
absorption
≤0.015 at 300 nm at 100 mg/mL
storage temp.
20-25°C
Other Notes
For life science research only. Not for use in diagnostic procedures.
The pH values of all buffers are temperature- and concentration-dependent. For Tris buffers, pH increases about 0.03 unit per °C decrease in temperature, and decreases 0.03-0.05 unit per ten-fold dilution.
For precise applications, use a carefully calibrated pH meter with a glass/calomel combination electrode.
For precise applications, use a carefully calibrated pH meter with a glass/calomel combination electrode.
Legal Information
Trizma is a registered trademark of Merck KGaA, Darmstadt, Germany

![Hydrochloric acid 2 mol/l (2 N) Titripur® [1 Litre]](https://idealmedical.co.za/wp/wp-content/uploads/2020/07/256-Hydrochloric-acid-200x200.jpg)


Reviews
There are no reviews yet.