Description
ACS reagent, ≥99.5%
Empirical Formula (Hill Notation): Br2
Molecular Weight: 159.81
General description
Bromine is a highly volatile and reactive halogen that can be used as a powerful brominating and oxidizing agent in chemical synthesis.
Application
Bromine can be used as a brominating agent for the bromination of:
- Aromatic and heterocyclic compounds.
- Carbonyl compounds at α position to carbonyl groups.
- Aromatic carboxylic acids via Hell-Volhard-Zelinski reaction in the presence of phosphorus trihalides.
- Alkylbenzenes at the benzylic position using photocatalytic conditions.
It can also be used as an oxidizing agent for the oxidation of:
- Primary alcohols to either aldehydes or esters.
- Secondary alcohols to ketones.
It can also be used as a Lewis acid catalyst to:
- Convert epoxides and CO2 to cyclic carbonates in a continuous flow system.
- Synthesize bis(indolyl)methanes by reacting indoles with carbonyl compounds.
PROPERTIES
grade
ACS reagent
vapor density
7.14 (vs air)
vapor pressure
175 mmHg ( 20 °C)
671 mmHg ( 55 °C)
Assay
≥99.5%
form
liquid
Pack Size
1 Litre
resistivity
7.8E18 μΩ-cm, 20°C
impurities
organic bromine compounds, passes test
≤0.001% I2
≤0.001% S compounds
evapn. residue
≤0.005%
bp
58.8 °C (lit.)
mp
−7.2 °C (lit.)
density
3.119 g/mL at 25 °C (lit.)
anion traces
chloride (Cl–): ≤0.05%
cation traces
Ni: ≤5 ppm
heavy metals: ≤2 ppm
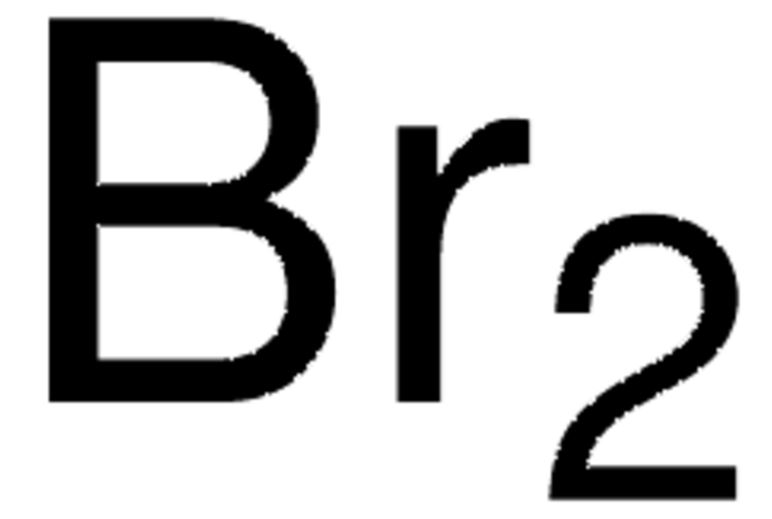




Reviews
There are no reviews yet.